Reducing the threat from terrorism and weapons of mass destruction and enhancing global strategic stability
LLNL develops innovative advanced technologies to help the government anticipate, identify, and address global security threats. By applying expertise in chemical, biological, radiological, nuclear, and explosive weapons, our researchers support threat preparedness, prevention, protection, and response and recovery. In addition, Livermore innovations in space situational awareness and cyberdefense help strengthen national security in an increasingly interconnected world.
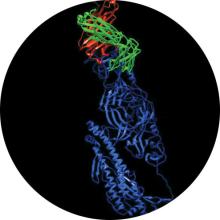
COVID-19 Antibodies and Diagnostics
In early February, Laboratory researchers rapidly responded to the COVID-19 crisis with a preliminary set of predicted 3D protein structures based on a previously peer-reviewed modeling process, the genetic sequence of SARS-CoV-2, and the known structure of a protein found in the virus that causes SARS. The predictions closely matched the later experimentally determined structures of the key protein. In continuing efforts, LLNL researchers have been searching for candidate antibodies capable of binding and neutralizing SARS-CoV-2. They are using a unique combination of artificial-intelligence virtual screening of antibodies to find possible candidates and simulations on world-class supercomputers to test the molecular interactions for efficacy. In a matter of weeks, this approach narrowed down the number of antibody candidates from 1039 possibilities to millions to about 20 initial candidates for synthesis and testing. LLNL’s COVID-19 research results are being shared with scientists worldwide through a searchable data portal.
Three Laboratory-developed technologies are improving the speed and accuracy of diagnostic tests for SARS-CoV-2. In early May, Bio-Rad Laboratories Inc. announced that its SARS-CoV-2 Droplet Digital PCR test kit, based on a technology licensed from LLNL more than a decade ago, had been granted authorization for emergency use by the U.S. Food and Drug Administration. Cepheid Inc., another LLNL licensee, also received emergency use authorization for a diagnostic test based on rapid PCR thermocycling. In addition, the Lawrence Livermore Microbial Detection Array (LLMDA), a DNA-based array that analyzes for more than 12,000 known/sequenced microbes, was rapidly updated to also detect SARS-CoV-2. Laboratory scientists are now using it, in collaboration with healthcare professionals, to analyze samples from COVID-19 patients and identify co-infections with other viruses or bacteria.
Vaccines Against Pathogens
Livermore is developing two vaccines based on an LLNL-developed biomedical technology, nanolipoprotein (NLP) particle platforms that can deliver vaccines and drugs inside the human body. NLPs are naturally occurring molecules that serve as structural mimics of cell membranes by self-assembling to provide a platform for connecting other molecules. The NLP platform makes it possible to develop customized vaccines to target a range of pathogens, including a broad-specturm coronavirus vaccine. With support from the National Institutes of Health, LLNL and two University of California campuses—Irvine and Davis—have teamed up to develop a vaccine for chlamydia, a sexually transmitted infection linked with ovarian cancer. In addition, the Laboratory and collaborators have developed a candidate vaccine for tularemia, a pathogen considered to be a biosecurity threat. The DOD Defense Threat Reduction Agency is supporting further development and testing to bring it to readiness for use.
Progress in Nuclear Nonproliferation
In FY 2020, the Low Yield Nuclear Monitoring program integrated explosion hydrodynamic codes with far-field energy propagation algorithms and atmospheric transport models and developed new machine learning methods. This advance resulted in improved understanding, prediction, and detection of seismic, acoustic, electromagnetic, and radionuclide signals from underground nuclear explosions. In addition, with LLNL leadership, the program began development of a test bed at the Nevada National Security Site to support integrated field experiments, which are designed to strengthen U.S. capabilities to detect and characterize low yield and evasively conducted underground nuclear explosions. Other efforts aimed to strengthen nonproliferation arms control regimes. During the pandemic, LLNL provided technical experts and presentations at two virtual meetings hosted by the Comprehensive Nuclear-Test-Ban Treaty Organization. In addition, Livermore scientists developed and participated in an interactive session at a ministerial-level event held by the International Atomic Energy Agency.
Brain-on-a-Chip in 3D
Laboratory researchers successfully created a 3D “brain-on-a-chip” microelectrode array platform in which they were able to keep hundreds of thousands of human-derived neurons alive, networked, and communicating in a 3D gel. The electrical spikes and bursts were non-invasively recorded for up to 45 days using LLNL-developed, thin-film microelectrode arrays. An important step beyond previously-developed 2D brain-on-a-chip platforms, a 3D culture model can more fully replicate the physiology and functionality of the human brain to understand how it functions and how chemicals or other stimuli can affect it. The team also developed a device capable of reproducing the blood–brain barrier (BBB) in both 2D and 3D, which could enable future devices to be more physiologically relevant. The BBB keeps potentially harmful molecules from entering and affecting the central nervous system but makes it difficult to get a therapeutic drug across the threshold. Laboratory scientists were able to introduce cells and nutrients into the device and remove waste products without stopping the flow. In addition to successfully creating the BBB, the researchers showed the barrier system was responsive.
Forensic Scientists Earn Two “A” Grades
In FY 2020, for the tenth straight year, scientists at the Laboratory’s Forensic Science Center (FSC) scored an “A” grade in the environmental proficiency test administered by the Organisation for the Prohibition of Chemical Weapons (OPCW). The Livermore researchers correctly identified all nine reportable spiked chemicals under the test’s scenario of samples collected from a laboratory accused of performing chemical weapons research. Since 2003, the FSC has maintained OPCW accreditation as a designated laboratory for sample testing. LLNL has also been an OPCW-designated laboratory for the analysis of biomedical samples since 2016, when the certification process started. In 2020, the FSC also passed its biomedical proficiency test with an “A” grade.
LLNL on the Watch at Mars Mission Launch
LLNL’s National Atmospheric Release Advisory Center (NARAC) and Nuclear Emergency Response Team have maintained full readiness during the pandemic. In July, two Laboratory scientists deployed to Cape Canaveral Air Force Station for the rocket launch sending the Perseverance rover to Mars with the rover’s plutonium-238 nuclear power source on board. They watched lift off from the radiological control center to respond in the unlikely event that a problem occurred during launch. Since 1989, NARAC scientists have supported eight NASA missions in which the spacecraft has carried a radioisotope thermoelectric generator.